2010
PROTEIN INTAKE AND CRISIS – WHAT WE HAVE DISCOVERED:
A Case Study
Our findings began with a call from a family whose FD child had been having frequent crises over a period of several months, which continued unabated despite the efforts of the treating physicians. Various medications had been tried, with no success. The parents contacted us and informed us that their child was taking the tocotrienols and green tea daily and was nevertheless still having crises on almost a daily basis. We reviewed the child’s regimen and food intake and could not find any tyramine-related triggers that could explain the crises. We were perplexed. We know that tyramine and tyramine-like molecules can cause crises, but this child’s diet appeared faultless. We asked ourselves, if he isn’t ingesting any triggering molecules, why was he in crisis? The mother suggested that it might be due to the start of puberty, but we have seen many children go through puberty without any problems and we therefore did not attribute the crises to this life change. One thing that we found somewhat unusual was that this child was getting rather large g-tube feeds every night. We suggested that the parents reduce the amount of the feeds, but this did not have any affect. The crises were unrelenting. We continued to focus on tyramine as being the trigger for the crises and asked ourselves how tyramine might be entering his body. One day we considered whether it was possible that the bacteria in the child’s gastrointestinal tract might be producing tyramine from the protein that was being ingested. We did a “search” using the terms “bacteria”, “intestine” and “tyramine” and soon came across the term “dysbiosis”, which is a condition of having microbial imbalances in the body in which the balance between beneficial and pathogenic microorganisms is disturbed (1-3). Intestinal dysbiosis has been demonstrated to result in the generation of tyramine from protein present in the digestive system (4-6). The tyramine that is generated by the bacteria in the intestine has been demonstrated to enter the circulatory system and cause hypertensive crises (7, 8). Additional investigation revealed that intestinal dysbiosis is encouraged by a condition referred to as hypochlorhydria, which is the reduced presence of stomach acid (9-11). The child in question, like so many other children with FD, takes medication to reduce the presence of stomach acid.
As we considered the matter further and were looking for some clue as to whether we were on the right track, we wondered whether the disruption of the bacteria in the digestive system (as a result of the use of antibiotics) might impact the frequency of this child’s crises. We contacted the mother and asked whether the child had been on an antibiotic during the past few months and whether the frequency or intensity of the crises had changed as a function of having taken the antibiotic. What we heard astounded us and strengthened our hypothesis. We were informed that about 24 hours after starting an antibiotic, the crises completely disappeared and did not return until after completing the course of antibiotics. Based on what we had learned, we hypothesized that the bacteria in the colon of this child were converting breakdown products of ingested protein into compounds such as tyramine which can be crisis-inducing for an individual with FD. Based on the observations we suggested to the mother that she might want to greatly reduce the amount of protein that this child received for a few days so that we could determine if the reduced protein intake might impact the frequency of the crises. The mother was concerned about what this restriction would do to the child’s weight, but as the crises were seriously compromising her child’s life, she decided to try a low protein diet for a few days. For the first few days, the mom eliminated all animal protein from the child’s diet. Within 48 hours, the child was crisis-free. The mom then reintroduced animal protein into her child’s diet but has kept his protein consumption to the Dietary Reference Intakes (DRI) recommended for a child his age (see table below as provided by the Food and Nutrition Board, Institute of Medicine, National Academy of Sciences).
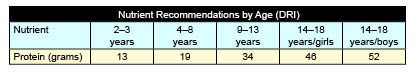
We are pleased to report that this child has remained crisis-free. Having made this observation in one child, we were unsure as to whether there was a real cause-and-effect or whether it was merely coincidence. Due to our research successes, we often receive calls from parents whose children are in crisis. Sometimes the triggers for the crises are easily discernable, while at other times the cause can be elusive. In the cases where there were no obvious triggers for an ongoing crisis and the children were taking advantage of the therapeutic regimens that we have developed, we have suggested that the amount of protein intake be reduced and, in almost every case, the crisis abated in a day or two. In addition, in many cases, parents have reported that their child is no longer manifesting the retching and nausea that were common every morning before the reduction in protein intake. For those children whose crises went away, but were still experiencing retching and nausea in the mornings, we suggested moving the protein-containing meal to earlier in the day so that their child was not going to sleep with a large amount of partially digested protein in the digestive tract. In these individuals as well, the periods of retching and nausea disappeared.
Bacteria play a very active role in the health of our colons. Bacteria make up about 60% of the dry mass of our feces and they are responsible for the processing of much of what we eat. There is clear evidence that the bacteria that reside in the colon are impacted by what we eat and that an imbalance in the flora that live in the colon can produce excessive amounts of tyramine and other compounds problematic for those with FD. It is interesting to note that we have for some time known that constipation is a trigger for crisis, though we never understood why it was having this effect. We now postulate that the delay in the elimination of feces is allowing the bacteria additional time to produce tyramine, which is in turn triggering crisis. For those whose children have been having crises, we suggest that their children not eat in excess of the recommended daily amount of protein. We also suggest that the protein-containing meal not be given near the end of the day, so that it doesn’t remain in the digestive system while they are sleeping when there is very little movement in the colon. For those children whose only source of nutrition is enteral formula, we suggest that you discuss the matter with the treating physician and consider using other formulations that have a lower protein content.
Additional points of interest
As the children with FD tend to be underweight, we were concerned that the reduction in protein intake might negatively impact their weight. What we found has surprised us. Many of the children who are now being limited to the Recommended Daily Allowance (RDA) of protein have actually put on weight and are achieving weights that they had never achieved before. When discussing this with the children, they have informed us that they are no longer going through periods of nausea and that they are eating more because they are hungry. Parents have commented that since reducing the protein intake of their children, the children have developed voracious appetites. We are not sure if the weight gain is due to the increased consumption of food or whether the metabolism of those with FD is negatively impacted by an excess amount of protein intake. Why the children are putting on weight is of less importance; what is important is that most of the children who are on the “Recommended protein allowance” diet are putting on weight. Parents who have put their children on this diet also have reflected on the fact that their children are in a better mood and that they appear to have a greater ability to focus. These observations are really not very surprising, as even small amounts of tyramine can negatively impact the health and well-being of a child with FD.
Other reasons to limit protein intake for a child with FD:
1) There have been reports in the literature by Axelrod and coworkers that state that chronic kidney disease is one of the complications of FD (12, 13). While it remains unclear as to why kidney disease is a complication of FD, what is clear is that excess protein intake causes kidney damage (see: Mayo Clinic). Is the chronic kidney disease observed by Axelrod and coworkers the result of the use of feeding formulas that contain excess amounts of protein? A review of the nutritional information of various formulas consumed by children with FD reveals, for example, that 1000 ml of Compleat contains 48 grams of protein, which is approximately 2.5 times the amount appropriate for a child 4-8 years old, and 1000 ml of Pediasure has 30 grams of protein, which is 50% more than the amount appropriate for a child 4-8 years old. In our view, it is possible that the use of feeding formulas that contain excess amounts of protein are contributing to kidney problems. Parents should discuss this issue with their child’s physician.
2) There is now clear evidence that the ingestion of excess protein results in the excretion of calcium from the body and can result in the development of osteopenia and osteoporosis (see: FWHC ). As osteopenia and osteoporosis have been observed in many of the children with FD, we wonder whether the calcium loss observed in these children is due to the excessive intake of protein.
Please note: Before making any change to your child’s diet, you should discuss this matter with her/his treating physician.
REFERENCES
1: Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut. 2004 53:1-4.
2: Hawrelak JA, Myers SP. The causes of intestinal dysbiosis: a review. Altern Med Rev. 2004 9:180-97.
3: Sartor RB. Therapeutic correction of bacterial dysbiosis discovered by molecular techniques. Proc Natl Acad Sci U S A. 2008 105:16413-4.
4: Perry TL, Hestrin M, MacDougall L, Hansen S. Urinary amines of intestinal bacterial origin. Clin Chim Acta. 1966 14:116-23.
5: Asatoor AM. The origin of urinary tyramine. Formation in tissue and by intestinal microorganisms. Clin Chim Acta. 1968 22:223-9.
6: Ladero V, Fernández M, Alvarez MA. Isolation and identification of tyramine-producing enterococci from human fecal samples. Can J Microbiol. 2009 55:215-8.
7: Hanke MT, Koessler KK. On the faculty of normal intestinal bacteria to form toxic amines. J. Biol. Chem. 1924 59, 835-853.
8: Coyle CL, Boyd TE. The absorption of tyramine from the intestine. Am J Physiol. 1932; 99: 317-320.
9: Husebye E. The pathogenesis of gastrointestinal bacterial overgrowth. Chemotherapy. 2005;51 Suppl 1:1-22.
10: Williams C, McColl KE. Review article: proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther. 2006 23:3-10.
11: Kanno T, Matsuki T, Oka M, Utsunomiya H, Inada K, Magari H, Inoue I, Maekita
T, Ueda K, Enomoto S, Iguchi M, Yanaoka K, Tamai H, Akimoto S, Nomoto K, Tanaka R, Ichinose M. Gastric acid reduction leads to an alteration in lower intestinal microflora. Biochem Biophys Res Commun. 2009 381:666-70.
12: Pearson J, Gallo G, Gluck M, Axelrod F. Renal disease in familial dysautonomia. Kidney Int. 1980 17:102-12.
13: Elkayam L, Matalon A, Tseng CH, Axelrod F. Prevalence and severity of renal disease in familial dysautonomia. Am J Kidney Dis. 2006 48:780-6.
